Product Labeling Symbols
| Symbol | Code | Standard | Ref # | Title | GS1 Application Identifier | Definition |
|---|---|---|---|---|---|---|
 |
3082 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.1 |
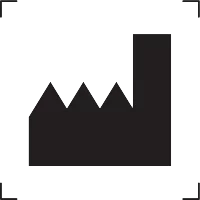
Manufacturer |
n/a |
Indicates the medical device manufacturer. |
 |
n/a |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.2 |
Authorized European Representative |
n/a |
Indicates the Authorized representative in the European Community. |
 |
2497 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.3 |
Date of Manufacture |
(11) |
Indicates the date when the medical device was manufactured. |
 |
2607 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.4 |
Use by date |
(17) |
Indicates the date after which the medical device is not to be used. |
 |
2492 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.5 |
Batch Code |
(10) |
Indicates the manufacturer’s batch code so that the batch or lot can be identified. NOTE: Synonyms for “batch code” is “lot number”. |
 |
2493 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.6 |
Catalogue number |
n/a |
Indicates the manufacturer’s catalogue number or part number so that the medical device can be identified. |
 |
2498 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.7 |
Serial Number |
(21) |
Indicates the manufacturer’s serial number so that a specific medical device can be identified. |
 |
6049 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.11 |
Country of Manufacture |
n/a |
To identify the country of manufacture of products. |
 |
2608 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.2.6 |
Do not resterilize |
n/a |
Indicates a medical device that is not to be resterilized. |
 |
2609 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.2.7 |
Non-Sterile |
n/a |
Indicates a medical device that has not been subjected to a sterilization process. |
 |
2606 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.2.8 |
Do not use if package is damaged and consult instructions for use |
n/a |
Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information. |
 |
0626 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.3.4 |
Keep dry |
n/a |
Indicates a medical device that needs to be protected from moisture. |
 |
0659 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.4.1 |
Biological risks |
n/a |
Indicates that there are potential biological risks associated with the medical device. |
 |
1051 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.4.2 |
Do not re-use |
n/a |
Indicates a medical device that is intended for one single use only. |
 |
1641 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.4.3 |
Consult instructions for use |
n/a |
Indicates the need for the user to consult the instructions for use. |
 |
0434A |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.4.4 |
Caution |
n/a |
Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. |
 |
3725 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.8 |
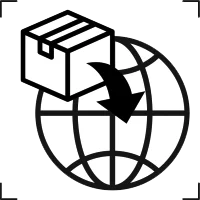
Importer |
n/a |
To indicate the entity importing the medical device into the locale. |
 |
3724 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.9 |
Distributor |
n/a |
Distributor |
 |
n/a |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.7.10 |
Unique Device Identifier |
n/a |
Indicates a carrier that contains unique device identifier information. |
 |
3728 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.7.7 |
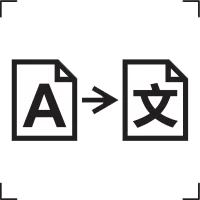
Translation |
n/a |
To identify that the original medical device information has undergone a translation which supplements or replaces the original information. |
 |
3727 |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.7.9 |
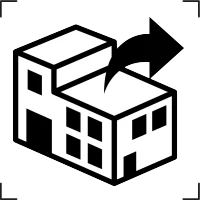
Repackaging |
n/a |
To identify that a modification to the original medical device packaging configuration has occurred. |
 |
n/a |
21 CFR 801.15 |
n/a |
RX Only |
n/a |
Caution: Federal (US) Law restricts this device to sale by or on the order of a physician. |
 |
n/a |
EU MDR 2017/745 and Regulation (EC) 765/2008 |
5.7.9 |
CE Mark |
n/a |
Signifies Euorpean Conformity. |
